
Active packaging is a primary concern when developing and packaging pharmaceutical products, particularly, when it comes to the sorbent, or desiccant, you use. Desiccants are an essential part of active packaging as they prevent degradation through moisture, extending the lifetime of the product.
While the cap/closure mechanism and the bottle will physically protect medicines, the type, quality and quantity of the desiccant used will also play a vital role in determining the quality of the formulation for the end-user. Use a generic desiccant and, in all likelihood, you won’t see the best results. In fact, the wrong type and quantity of desiccant can result in over-desiccation of the formulation and may be detrimental to patient safety.
So you can see the importance of choosing your desiccant carefully. The cheapest, or most convenient option may not be the most valuable solution.
Use a desiccant that’s fit for purpose
At CILICANT, we understand how critical it is to choose the right desiccant. That’s why we offer a variety of sorbents developed for different applications. The two most commonly used are silica gel and molecular sieve.
Significantly, the moisture adsorption properties of molecular sieve and silica gel differ when it comes to how they perform. While molecular sieve has an excellent adsorptive capacity at low humidity levels, silica gel tends to perform poorly in low humidity. Both, however, have similar moisture retention capacities at room temperature (25°C) at a relative humidity of 40%.
Silica gel is the most suitable desiccant to use in products with stable storage temperatures and high relative humidity. With its inert, non-toxic, and highly stable properties, understandably, silica gel is a popular desiccant choice for many pharmaceutical products. 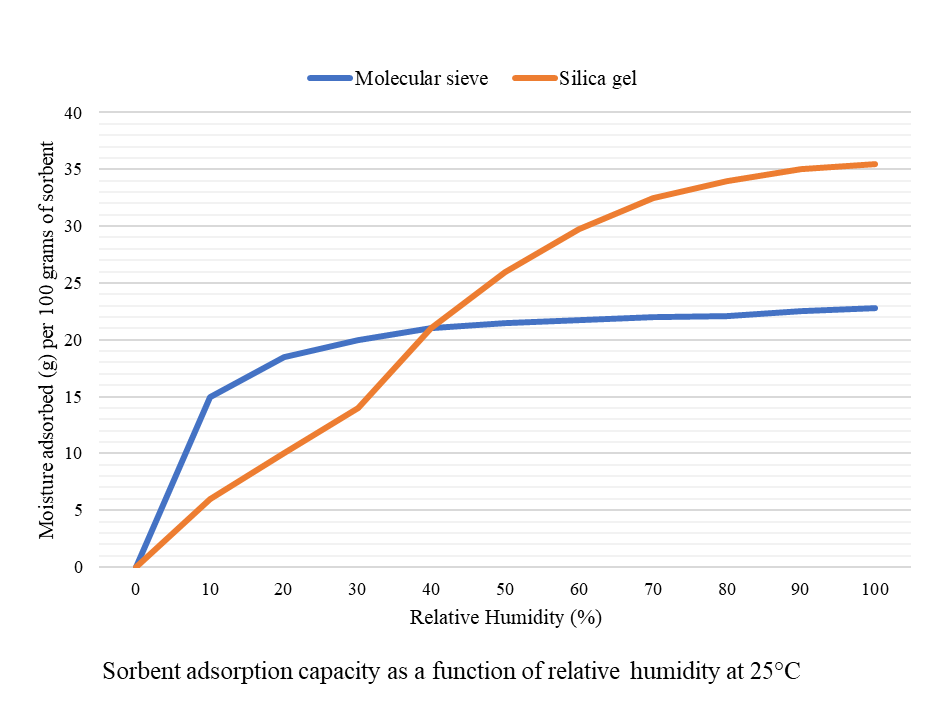
On the other hand, molecular sieve is more aggressive and rapid when it comes to moisture adsorption, so performs better in products that require instant protection from moisture degradation. Molecular sieve is also stable over a large temperature range maintaining its higher moisture retention properties. However, caution should be exercised when selecting the proper dosage for use in products with specific RH requirements.
Packaging specialists also need to consider the following in order to get optimal results from the desiccants they use.
While choosing the right adsorbent is important, it’s equally important to choose the right product within the desiccant range, as each serves different purposes. In the case of molecular sieve, there are several variants commonly used, including 3A, 4A and 13X. Type 4A is a popular choice for pharmaceutical packaging.
Likewise, there are several types of silica gel. Type A, B, C are the most common. Here, type A has been specifically designed for the adsorption of moisture in pharmaceutical packaging.
To get the optimum results from the desiccant one selects, users should examine a number of testing parameters, such as LOD (loss on drying), MAC (Moisture Adsorption Capacity) at low RH and high temperatures, along with other parameters such as packaging headspace and the regional climate at the product’s destination, etc.
Clearly, inert products, such as medical devices, diagnostic kits and API’s will be more compatible with silica gel, while thermal-sensitive medicines will be protected better by using molecular sieve desiccants. As both over-desiccation, as well as under-desiccation, can impact the life span and efficacy of formulations, companies involved in development and packaging of these products need to ensure that they’re using the most appropriate form of adsorbent technology.
For more information on the best desiccants for your product, get in touch with our technical sales representative now!
“The information provided by Cilicant Private Limited in this paper is for general informational purposes only and shall not be relied upon by anyone for any other purpose. We make no representation or warranty of any kind, express or implied, regarding the accuracy, adequacy, validity, reliability or completeness of any information provided in this paper. Cilicant Private Limited owns all the intellectual property rights in the contents of this paper. The contents of this paper shall not be replicated or reproduced in any manner whatsoever without the prior written permission of Cilicant Private Limited. Cilicant Private Limited shall not be liable for any direct, indirect, incidental, consequential or punitive damages or losses resulting from your reliance on the data provided in this paper.”



